Electronic prescribing has become the default in most healthcare settings, not because it’s trendy, but because the old way simply couldn’t keep up. Handwritten scripts, fax machines, and manual phone verifications introduced too much room for error, delay, and misuse.
But moving prescribing online only works if everyone, providers, pharmacies, and payers, speaks the same language. That’s where standards come in.
Two of the most critical are NCPDP SCRIPT, which defines how prescription data is structured and shared, and EPCS, which adds the security layer needed to safely prescribe controlled substances. One ensures interoperability. The other ensures trust.
Together, they form the backbone of a prescribing system that’s not just digital, but dependable. In this blog, we’ll unpack what these standards actually do, why they’re required, and what it takes to implement them in real-world healthcare workflows.
1. Understanding NCPDP SCRIPT: Ensuring Interoperability
When an ePrescription moves from a provider’s EHR to a pharmacy’s system, it needs more than just a delivery channel; it needs a shared structure. The NCPDP SCRIPT standard provides that structure, acting as the common language for electronic prescribing across the U.S. healthcare system.
Developed by the National Council for Prescription Drug Programs (NCPDP), SCRIPT defines how prescription data should be organized, transmitted, and interpreted. Its scope goes beyond sending new prescriptions; it includes renewals, changes, cancellations, medication history, and prior authorization support. It’s designed to support the entire prescription lifecycle.
Key Features of NCPDP SCRIPT
- Structured Prescription Data
SCRIPT organizes every element of a prescription drug name, dosage, SIG, quantity, refills, prescriber ID, and pharmacy details into a machine-readable format, eliminating guesswork and reducing manual data entry.
- Support for Multi-directional Workflows
It enables pharmacies to request refills, send change requests, or respond to cancellation messages — not just passively receive prescriptions.
- Versioning to Match Clinical and Regulatory Needs
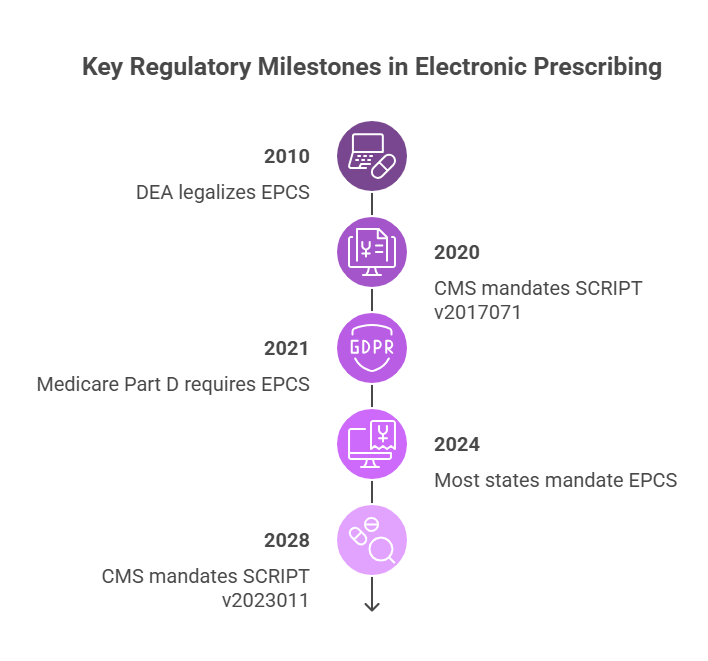
The most current required version, 2017071, has been mandated by CMS for Medicare Part D ePrescribing since 2020. It introduced expanded support for clinical coding systems and improved message flexibility across different prescription types.
Why SCRIPT Matters
Without SCRIPT, each EHR and pharmacy would need to maintain custom integrations with every other system — an impossible task at the national scale. SCRIPT eliminates that complexity by creating a predictable, reliable format for transmitting prescriptions across diverse platforms.
For providers, it reduces rework and follow-up calls. For pharmacies, it shortens fulfillment time. And for patients, it means fewer delays in accessing the medications they’ve been prescribed.
2. Delving into EPCS: Securing Controlled Substance Prescriptions
Prescribing controlled substances brings a different level of responsibility. Beyond dosage and drug interactions, providers need to consider security, traceability, and regulatory compliance. That’s where EPCS — Electronic Prescribing of Controlled Substances — plays a critical role.
Established and regulated by the U.S. Drug Enforcement Administration (DEA), EPCS enables providers to transmit prescriptions for Schedule II through V drugs electronically, under a tightly controlled framework. While ePrescribing in general improves speed and accuracy, EPCS adds the safeguards needed to prevent misuse, forgery, and diversion of opioids and other high-risk medications.
What EPCS Requires
To comply with DEA requirements, any system handling controlled substances must include several security and validation mechanisms:
- Two-Factor Authentication
Prescribers must authenticate using two credentials: something they know (e.g., a password or PIN) and something they have (e.g., a token, smart card, or mobile device). This step ensures that only authorized individuals can issue a controlled prescription.
- Identity Proofing
Before gaining access to EPCS features, prescribers must go through an identity verification process conducted by an approved Credential Service Provider (CSP) or Registration Authority (RA). This process links the prescriber’s digital identity with their DEA registration.
- Audit Logging
Every prescribing action is logged — not just the prescription itself, but the prescriber’s login, authentication event, and transmission timestamp. These logs are critical for internal monitoring and external audits.
- EHR and eRx System Certification
Only systems that meet DEA EPCS specifications and are certified accordingly can legally support electronic transmission of controlled substances. Certification covers both functional compliance and security controls.
The Benefits of EPCS
- Reduces Prescription Fraud
Electronic transmission cuts out paper prescriptions, which are susceptible to theft, alteration, or forgery. With EPCS, there’s no handwriting to manipulate, no physical form to intercept.
- Closes Diversion Loopholes
EPCS helps monitor and control the volume and frequency of controlled substance prescriptions, making it harder for patients to obtain multiple scripts from different providers.
- Supports State and Federal Compliance
As of 2024, the majority of U.S. states have adopted laws requiring the use of EPCS for all controlled substances. Additionally, Medicare Part D mandates that all controlled substances be prescribed electronically under CMS guidelines.
- Improves Pharmacy Confidence
Pharmacies receiving EPCS-enabled scripts know the prescription originated from a validated provider, through a certified system, and with full traceability, reducing their own verification burden.
EPCS Adoption Trends
Adoption of EPCS has accelerated over the past few years due to state mandates, CMS requirements, and rising pressure to reduce opioid misuse. According to Surescripts, over 300 million controlled substance prescriptions were transmitted electronically in 2023, marking a continued upward trend. However, many smaller practices still face challenges with onboarding and compliance, especially when EHR upgrades or credentialing processes are delayed.
3. The Synergy Between NCPDP SCRIPT and EPCS
NCPDP SCRIPT and EPCS are often discussed separately, one as a technical standard, the other as a regulatory framework. But in practice, they work best when implemented together. SCRIPT defines how prescription data is structured and transmitted. EPCS ensures that certain prescriptions, specifically controlled substances, are created and transmitted securely.
Used together, they form the foundation of a compliant and interoperable ePrescribing workflow.
Complementary Functions, One Workflow
- SCRIPT Handles the Data
It formats every detail in a consistent, machine-readable structure that EHRs, pharmacies, and intermediaries understand. It supports full-cycle transactions, from new scripts to refill requests and cancellations.
- EPCS Secures the Transaction
It governs who can send controlled substance prescriptions, how they are authenticated, and how those transactions are logged and verified.
Together, they support a complete, standards-driven prescribing flow:
- A provider writes a controlled medication in the EHR
- SCRIPT structures the data for transmission
- EPCS ensures the provider is authorized and authenticated
- The prescription reaches the pharmacy with the necessary safeguards and formatting to be processed without delay or error
Eliminating Gaps Between Format and Access
Without SCRIPT, systems would struggle to interpret prescription data consistently. Without EPCS, even well-structured controlled substance prescriptions could be subject to fraud or misuse. It’s the combination of the two that enables a prescribing system to be both interoperable and compliant, capable of working across platforms while satisfying DEA, CMS, and state-level requirements.
Streamlining Compliance Without Adding Complexity
When integrated properly, EHR and ePrescribing systems can use both standards in the background without adding steps for the provider. Multi-factor authentication happens quickly. Data is formatted automatically. Providers work within their normal clinical workflow, and the system handles the complexity behind the scenes.
This synergy is essential not only for compliance but for building a prescribing ecosystem that is fast, secure, and scalable, especially in environments handling high volumes of controlled medications, such as pain management clinics, behavioral health, and long-term care.
4. Regulatory Landscape and Compliance
The push toward standardized and secure ePrescribing isn’t just a best practice — it’s now a regulatory expectation. Multiple federal and state-level mandates require the use of standards like NCPDP SCRIPT and EPCS, making compliance non-negotiable for providers and technology vendors alike.
CMS Mandates
The Centers for Medicare & Medicaid Services (CMS) has played a key role in accelerating adoption. Since January 1, 2020, CMS has required the use of NCPDP SCRIPT version 2017071 for all Medicare Part D ePrescriptions. This version supports expanded data fields, structured SIG (directions), and more flexible prescription types, ensuring prescriptions sent to Medicare beneficiaries are consistent, complete, and processable by pharmacies.
CMS has also enforced mandatory electronic prescribing for controlled substances under Medicare Part D since 2021, aligning federal requirements with broader digital health initiatives. Providers who fail to comply may face penalties or reimbursement delays, particularly in high-volume prescribing environments.
DEA Regulations
The Drug Enforcement Administration (DEA) governs the use of EPCS nationwide. DEA regulations specify how providers must prove their identity, how systems must log and secure prescribing actions, and what technical safeguards need to be in place for systems to be certified.
EPCS compliance is not optional for any healthcare organization prescribing controlled substances electronically. It applies equally to large hospital networks and solo outpatient clinics.
State-Level Requirements
Beyond federal oversight, individual states have passed laws mandating EPCS for all controlled substance prescriptions, many with strict implementation deadlines. As of 2024, nearly every U.S. state requires or strongly encourages electronic prescribing of opioids and other scheduled medications. These laws are often tied to broader efforts to combat prescription drug abuse and reduce diversion.
States may also impose their own reporting requirements, integrating EPCS data with Prescription Drug Monitoring Programs (PDMPs), which track controlled substance prescriptions across providers and pharmacies.
The Compliance Expectation for EHR Vendors and Providers
For healthcare software vendors, compliance isn’t just about ticking boxes — it’s about maintaining certification under programs like the ONC Health IT Certification Program. Without certification, providers using the software risk falling out of alignment with CMS requirements.
For providers, maintaining compliance means ensuring their prescribing systems are configured correctly, their users are credentialed and trained, and their data exchange workflows follow both federal and state guidelines.
5. Challenges in Implementing Standards
While the value of standards like NCPDP SCRIPT and EPCS is clear, implementing them across diverse healthcare environments can be anything but straightforward. From technical complexity to organizational inertia, there are several challenges that slow or complicate adoption.
Technical Integration
Integrating NCPDP SCRIPT and EPCS into existing EHR or pharmacy management systems often involves significant engineering work. SCRIPT transactions must be mapped correctly, version support must be verified, and updates need to be regression-tested within clinical workflows. Systems that were built before SCRIPT became mandatory may require structural changes just to support newer transaction types or message formats.
For EPCS, integrating multi-factor authentication and audit logging into prescribing workflows requires not only technical upgrades but also third-party services for credentialing, identity proofing, and compliance auditing. Ensuring all components meet DEA requirements can be a multi-month project, especially for vendors supporting legacy infrastructures.
Credentialing and Access Control
EPCS implementation introduces new administrative layers. Providers must undergo identity proofing before they can prescribe controlled substances electronically, and access must be managed securely within the organization. Larger health systems often need to coordinate with credentialing departments, pharmacy teams, IT, and compliance officers, adding complexity to rollout plans.
For smaller practices, these steps can feel disproportionate to the size of the operation, making adoption seem out of reach without outside support.
User Training and Workflow Adoption
Even the best technical implementation can fail without proper user adoption. Prescribers need to understand not just how to send an electronic prescription, but what to look for in the workflow — formulary mismatches, structured SIGs, approval queues, and EPCS-related sign-offs.
If training is rushed or incomplete, errors increase, and confidence in the system drops. This can lead providers to revert to manual workarounds, like phone calls or printouts, even when the electronic system is available.
Ongoing Maintenance and Version Support
SCRIPT isn’t static. As CMS mandates new versions or the NCPDP releases updates, vendors must revalidate their workflows and perform regular testing. Similarly, changes to DEA rules or state-level EPCS requirements can trigger system updates, new authentication processes, or policy changes within provider organizations.
Maintaining compliance isn’t just about implementation — it’s about staying aligned as the regulatory and technical environment evolves.
6. The Future of ePrescribing Standards
As healthcare continues its digital transformation, ePrescribing standards are evolving to meet new clinical, regulatory, and technological demands. The forthcoming updates to NCPDP SCRIPT and EPCS reflect a concerted effort to enhance interoperability, security, and efficiency in medication management.
NCPDP SCRIPT Version 2023011: Enhancing Interoperability
The NCPDP SCRIPT Version 2023011 is set to become the mandated standard for Medicare Part D ePrescribing by January 1, 2028. This version introduces several enhancements:
- Three-Way Communication Support: Facilitates improved coordination among prescribers, pharmacies, and long-term care facilities, addressing previous limitations in communication workflows.
- Expanded Data Elements: Incorporates additional fields to support comprehensive medication management, including detailed patient instructions and structured sigs.
- Enhanced Support for Electronic Prior Authorization (ePA): Streamlines the ePA process, reducing delays in medication dispensing.
These improvements aim to bolster the accuracy and completeness of prescription data, thereby enhancing patient safety and care continuity.
EPCS: Strengthening Controlled Substance Prescribing
The Electronic Prescribing of Controlled Substances (EPCS) framework continues to be integral in combating prescription fraud and abuse. Future developments in EPCS are expected to focus on:
- Advanced Authentication Methods: Adoption of biometric verification and other robust authentication mechanisms to ensure only authorized prescribers can issue controlled substance prescriptions.
- Integration with Prescription Drug Monitoring Programs (PDMPs): Enhanced interoperability with state-run PDMPs to provide real-time data on controlled substance prescriptions, aiding in the identification of potential abuse patterns.
- Improved Audit Trails: Implementation of more detailed logging mechanisms to facilitate compliance monitoring and auditing processes.
These advancements are designed to fortify the security of controlled substance prescribing and align with evolving regulatory requirements.
Conclusion: The Imperative of Adhering to ePrescribing Standards
Standards like NCPDP SCRIPT and EPCS are more than regulatory checkboxes — they form the foundation of a safe, interoperable, and efficient ePrescribing ecosystem. Together, these standards ensure prescriptions are transmitted clearly, securely, and compliantly, protecting patients and providers alike.
With evolving mandates from CMS, DEA, and state agencies, healthcare organizations must stay proactive in adopting and maintaining these standards. The complexity of implementation, while challenging, is necessary to keep pace with a healthcare system that demands accuracy, security, and seamless communication.
At Nalashaa, we specialize in helping healthcare providers and technology partners navigate this evolving landscape. From integrating NCPDP SCRIPT workflows and ensuring EPCS compliance to optimizing refill management, our healthcare software solutions simplify the complexities behind ePrescribing.
If your organization is ready to build or modernize its digital prescribing capabilities, we’re here to help. Reach out to us at info@nalashaa.com to start the conversation.
Latest posts by Priti Prabha (see all)
- ONC HTI 1 Final Rule and Certified APIs: A Complete Guide - February 19, 2026