In a world where technology has become an important aspect of everyone’s life, there are not many industries that can stay away from adopting new technology. Healthcare is one of it. Although technology has majorly taken over different aspects of healthcare, there are some remarkable changes that still take place. One of the changes that gained momentum in recent days is entry of Remote Patient Monitoring device into Medicare.
Remote Patient Monitoring is a method of healthcare delivery that uses the latest advances in information technology to gather patient data outside traditional healthcare settings. This part of healthcare was not included under Medicare till now as it involved technology and telecommunications system. Previously, Medicare beneficiaries were eligible for tele-health service only if they were presented from an originating site, located in either rural Health Professional Shortage Area or in a county outside a Metropolitan Statistical Area. Patient’s home was not considered as an originating site.
Social Security Act had stated that tele-health services were outside the scope of Medicare Home Health Benefit. This means that the provision was not providing coverage or payment for Medicare Home Health Services provided via telecommunications system.
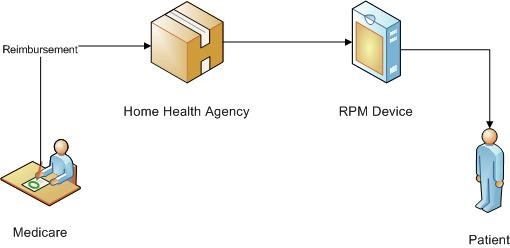
The new initiative taken by CMS takes this aspect of healthcare into consideration. According to the Final Rule, levied by CMS on 31st October, 2018, Remote Patient Monitoring devices are brought under the ambit of Medicare. This implies that Medicare will also pay for the cost involved in purchasing RPM devices by HHA or by Patients. Medicare, by including RPM in cost report is working in reducing the cost involved in RPM services.
In Final Rule, according to section III.H, “remote patient monitoring” has been brought under the Medicare Home Health Benefit and changes to the regulations at §409.46 to include costs of Remote Patient Monitoring as allowable administrative costs. In the statute, this regulation is defined as the collection of physiological data, digitally stored and transmitted from patient or caregivers to the HHA.
Although this change is positively greeted among the community, there also lays some concerns. One of the major concerns is that RPM devices should not be frequently used as a substitute for face to face visit. It means that in order to ensure that RPM is not being taken for granted, CMS should monitor the utilization pattern. CMS should also collect data about the duration and frequency of use of RPM service.
The regulation also ensures that the RPM service cannot be reported without the provision of another skilled service. This implies that the visit to a patient’s home, for training about RPM device or installing RPM device will not be accounted separately.
Advantages of this act:
- Quality of service will be improved
- More patients will be attracted towards Medicare as compared to the commercial plans
- Home Health Agencies can identify patients’ condition more promptly and make changes in care plan, if necessary
Conclusion:
This is one of the breakthrough moves by CMS. Although there are some areas where CMS needs to lay down certain supportive regulation, this move can be a game changer in US healthcare industry.
To know more about this initiative and its implications, you can reach out to us at info@nalashaa.com
Latest posts by Keerthi Chavva (see all)
- The CQL Mandate:- Here’s What Providers Need to Know - August 14, 2019